A new report from Avalere finds that under current law Medicare beneficiaries are likely to pay more for biosimilars than for the biologic reference product in Part D. For instance, for a sample drug costing $30,000 per year, patient costs for a biosimilar would be over $1,500 more per year (39 percent higher) than the reference product. Higher patient out-of-pocket costs may discourage use of biosimilars in Part D, reducing overall savings to the Medicare program.
Effects of Health Reform on Medicare Part D and Biosimilars
As part of the Affordable Care Act (ACA), Congress enabled the Food and Drug Administration (FDA) to create an approval pathway for biosimilars. To date, the FDA has approved two biosimilars in the United States. The launch of these and other biosimilars has the potential to generate savings to patients and the healthcare system because these products are expected to be priced lower than the reference product. For instance, in Europe, the average price discount for a biosimilar is 25 percent less than the reference product.
In addition to creating the biosimilar pathway described above, the ACA initiated a process to eventually close the Part D coverage gap by requiring manufacturers to provide patients discounts for branded drugs purchased in the “donut hole.” These discounts do not extend to biosimilars, which can result in increased beneficiary costs.
“The unintended consequence of the ACA is that consumers have a financial disincentive to switch to a lower-cost biosimilar,” said Caroline Pearson, senior vice president at Avalere. “While the Medicare program will save money if beneficiaries take biosimilars, higher consumer out-of-pocket costs are a barrier to patient adoption.”
Policy Solutions to Lower Consumer Costs
The Avalere report examines two potential policy options to reduce consumer costs for biosimilars:
Requiring manufacturer discounts to close the coverage gap for biosimilars, consistent with current law for branded drugs; and
Creating a biosimilar tier that would reduce beneficiary costs for the biosimilar below that of the reference product.
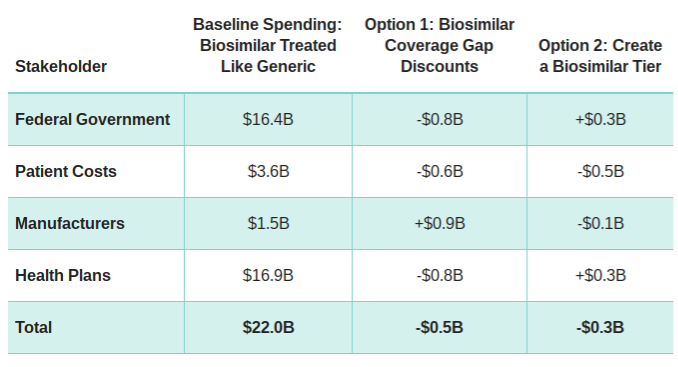
Based on Avalere’s analysis, both options could increase use of biosimilars and would decrease consumer costs. One option, requiring manufacturer discounts for biosimilars, would reduce federal spending by $800 million over 10 years, while increasing manufacturer costs. Another option, creating a biosimilars tier, would increase federal spending by $300 million over 10 years as a result of higher costs to the Part D benefit. A summary of total costs across stakeholders is shown below.
10-Year Cost Estimate for Different Policy Options, 2016-2025
“The decision to switch to a biosimilar medication is complex. Patients and physicians should work together to consider potential options,” said Gillian Woollett, senior vice president at Avalere. “Policymakers should explore options to share the financial benefit of increased use of biosimilars for the government with consumers.”
Avalere’s complete analysis is available here.
Methodology
Avalere included six reference products in its model, which account for the drugs with the highest spending in Medicare Part D that are likely to have biosimilars in the next 10 years. We assume biosimilars will have a list price 25 percent below the price of the reference product based on the current average cost per patient. The model does not account for discounts or rebates on either the reference product or the biosimilars. Under the baseline, adoption of the biosimilar product begins at 5 percent of the utilization for reference product and increases to a ceiling of 50 percent by year 6. We do not assume any therapeutic switching from other competitor biologics to the biosimilar. Options 1 and 2 assume higher rates of switching from the reference product to the biosimilar due to reduced consumer out-of-pocket costs for the biosimilar. For a complete discussion of our methodology, please see the full report linked above.